
|
Only have a minute? Listen instead
Getting your Trinity Audio player ready...
|
DHR Health is among the many healthcare and higher education institutions around the nation that are conducting a clinical trial that tests the effectiveness of inhalable insulin for children with Type 1 or Type 2 diabetes.
Throughout the trial, these facilities will be testing the Afrezza insulin inhaler which has already proven effective in adults, local medical experts said.
According to Dr. Adrienne Casciato, research coordinator for the inhale trial at DHR Health, the goal of the study is to determine how the Afrezza insulin inhaler affects children ages 4 to 17.
She explains that the inhaler will function the same as injected insulin due to it being a short-acting insulin, which begins to work quickly.
Casciato continued to explain that one of the major differences between an injection and an inhaler is that sometimes the effects of injected insulin can only carry up to 20-30 minutes after a meal, meaning the possibility of a child having a low-sugar episode increases if they do not eat enough.
With the Afrezza, which is an “ultra-short-acting insulin,” she hopes that parents will be able to prevent that.
She described the device as a small whistle that is entirely made of plastic, making it more portable, along with the insulin cartridge which is about the size of a Lego and which can be easily disposed of without the need for a sharps container.
With the inhaler, there would also be no need to refrigerate the medication.
“It makes their life a whole lot easier so that these kids can live a normal life,” Casciato said. “They can go on vacation, they can go to summer camp, they don’t have to go to the nurse during lunch and get injected.”
In addition to the inhaler being a less invasive form of diabetes treatment, Dr. Surya Narayan Mulukutla said it’s costlier than most other medicines due to how new it is and it lacking FDA approval.
“Insurance companies typically are unlikely to approve something that is not FDA approved,” Mulukutla said.
The inhaler would also eliminate the need to buy items such as needles, alcohol wipes or other products necessary with injection-based treatments.
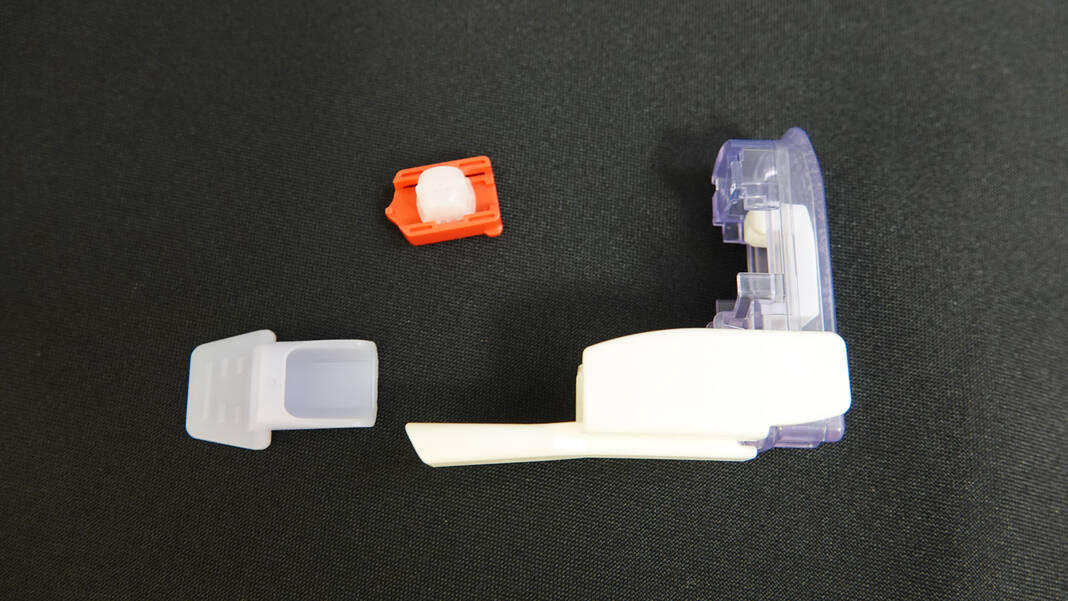
“(It would) reduce the number of insulin pokes that the kids get because now they’re inhaling insulin and also to reduce the number of times that their blood sugar drops a day,” Casciato said, adding that she hopes it will be a more stable form of insulin for children.
The clinical trial which is sponsored by Mankind Pharma, a pharmaceutical company, will run for a year for those who participate in the study.
Dr. Lisa Treviño, vice president of research operations at DHR Research Institute, explained the noninvasive nature of the medication is what gained DHR’s attention.
“I think this drew particular attention to our pediatric endocrinologist, … because it would be a lot easier for the mommies or the parents and the kids to be compliant,” Treviño said. “It’s an inhalable so it’s easier, it’s quicker and you don’t have to worry about calming the child down to get an injection.”
Criteria for those wishing to participate in the trial requires a child to have been diagnosed with Type 1 or Type 2 diabetes, and who have been receiving insulin for six months for the former’s treatment or three months for the latter’s.
Non-pregnant teenagers can also participate but cannot have asthma or any lung problems, and they cannot smoke or vape or be around anyone who smokes or vapes.
Other criteria includes passing a pulmonary function test during a screening process, which will also measure their breathing to determine if their lungs meet the capacity to receive the dosage.
A blood test to check hemoglobin A1C is also required and measures blood sugar for the last three months to ensure it falls between 7 and 11. Those whose blood sugar is too high or too controlled don’t qualify.
And they cannot be taking other medications that may harm them or interfere with the inhaler medication.
Treviño explained that the criteria’s in place to help ensure the safety of those participating.
The trial is still in the recruiting process as Casciato explained that DHR will be enrolling four children in the next couple of weeks.
Those wishing to participate in the trial can contact Casciato at (956) 362-2397 or via email at [email protected].
Editor’s note: This story was updated to include contact information for the trial.



